orange book pharmacy ab rating
Search for Service - Analytical - All Analytical - Analytical Method Development - All Analytical Method Development - Biologic Drugs - Capillary. Orange book pharmacy ab rating Monday August 29 2022 Edit.

Approved Drug Products With Therapeutic Equivalence Evaluations T H E O R A N G E B O O K Ppt Download
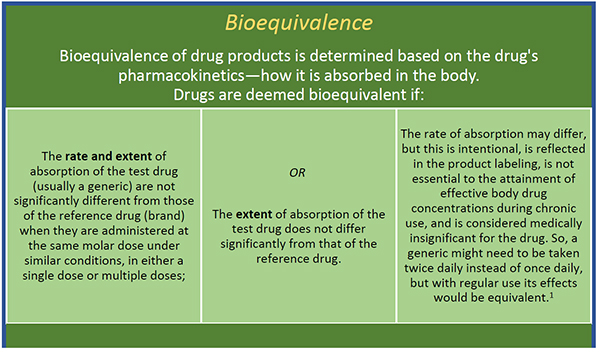
A generic medication with an AB rating has in vivo or in vitro study results proving that it is therapeutically equivalent displaying bioequivalence and.

. As such it is essential that pharmacists practicing in New York State have a thorough understanding of the Orange Book and the. Formally called Approved Drug Products with Therapeutic. Discuss the role that the ratings system is expected to play in pharmacy practice.
Find Suppliers APIFDF Services. A single source product that is brand only with no generic available for substitution Drug A is rated AB1. With respect to the dilemma concerning Cardizem CD noted earlier a search of the Orange Book revealed that Cardizem CD 240 mg is rated AB3.
Drugs rated as AB1 are bioequivalent and pharmaceutically equivalent to each other as are drugs that are AB2-rated and so on. What is AB rating pharmacy. The electronic Orange Book provides.
Generic Substitution Laws. Orange Book Rx Wiki Adhd Medication Options Stimulants Nonstimulants. A list of drugs that the US.
Thus generic products rated. Not listed in Orange Book. It has come to the Boards attention that one manufacturer has received an AB Rating for their Levothyroxine product.
The first letter -- A or B -- indicates. For more information on the Orange Book including its history see the Orange Book Preface. Search the Orange Book Database.
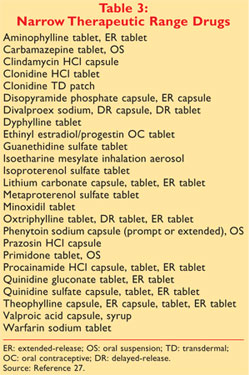
Pharmacists should be aware that the Narrow Therapeutic Index Drug. Food and Drug Administration FDA has approved as both safe and effective. Orange Book in choosing drugs for generic substitution.
Search approved drug products by active. Like prenatal vitamins ZC. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been.
Every drug listed in the Orange Book has a 2-letter code. Approved Drug Products with Therapeutic Equivalence Evaluations. The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book identifies drug products approved on the basis of.

Approved Drug Products With Therapeutic Equivalence 39th Edition 2019 U S Government Bookstore

Fda Offers New Guidance On Therapeutic Equivalence Evaluations Raps

Top Medications Flagged For Unauthorized Substitutions Paas National
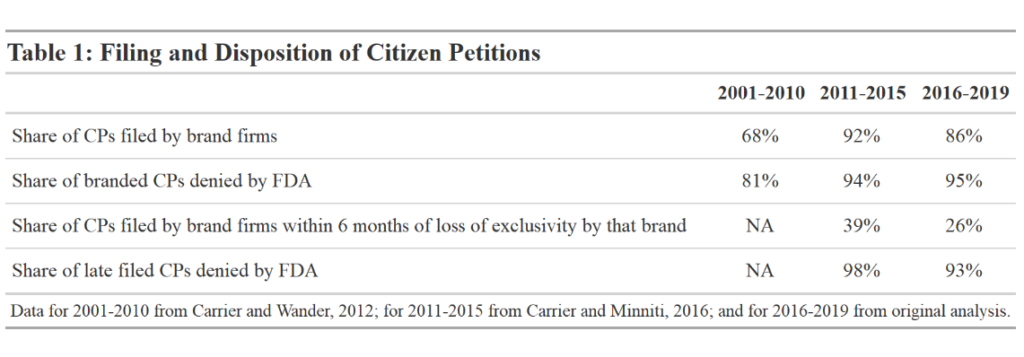
The Fda Could Do More To Promote Generic Competition Here S How Usc Schaeffer

Facts And Comparisons Lexicomp Wolters Kluwer

Approved Drug Products With Therapeutic Equivalence Evaluations T H E O R A N G E B O O K Ppt Download

Pharmacy Empower Healthcare Solutions Llc
Module 9 Generic Drugs And Therapeutic Equivalence

Medications That Require Special Handling Ppt Video Online Download

Drug Pricing And Pharmaceutical Patenting Practices Everycrsreport Com

Therapeutic Equivalence Codes Effects Substitution Video Lesson Transcript Study Com

Module 9 Generic Drugs And Therapeutic Equivalence
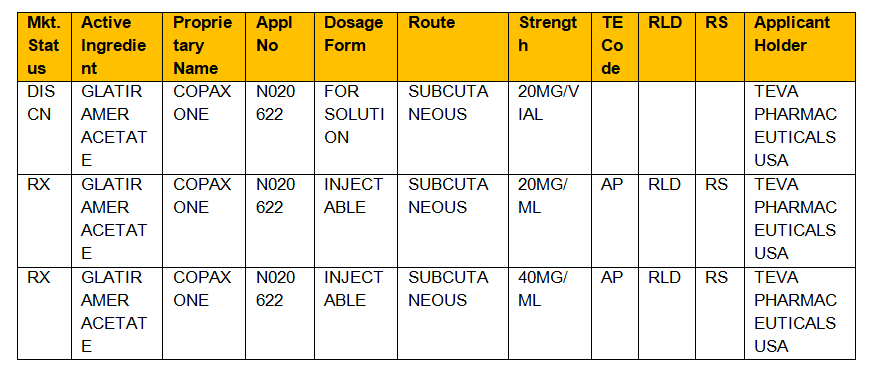
Orange Book And Its Applications Legal Advantage
The Fda Could Do More To Promote Generic Competition Here S How Usc Schaeffer